‘IP (Intellectual Property)’ Category
-
Teva v Gilead – C-121/17 provides some clarity on combination products
0July 26, 2018 by IPAlchemist
The CJEU gave its ruling yesterday in case C-121/17 in the case of Teva v Gilead. This concerns the SPC for the combination of tenofovir and emtricitabine (marketed by Gilead as Truvada ®), which was granted in the UK and which Teva and others were seeking to revoke. There was no SPC for the tenofovir alone, as far as I can tell, because the EU marketing authorisation for the mono product was granted within 5 years of the application date of the basic patent EP 0915894. Gilead has successfully argued that the patent, which did not mention emtricitabine, could serve as the basic patent for a combination SPC following authorisation of the combination, because claim 27 referred to:
A pharmaceutical composition comprising a compound according to any one of claims 1-25 together with a pharmaceutically acceptable carrier and optionally other therapeutic ingredients.
In proceedings in the Patents Court, Mr Justice Arnold considered that the CJEU jurisprudence on combination SPCs was still insufficiently clear, so he referred yet another question on Article 3(a) of the SPC Regulation to the CJEU:
‘What are the criteria for deciding whether “the product is protected by a basic patent in force” in Article 3(a) of Regulation No 469/2009?’
He offered a view – that it related to the inventive advice or technical contribution of the patent – stating at [97]:
In my view, the answer is that the product must infringe because it contains an active ingredient, or a combination of active ingredients, which embodies the inventive advance (or technical contribution) of the basic patent. Where the product is a combination of active ingredients, the combination, as distinct from one of them, must embody the inventive advance of the basic patent.
The Advocate General disagreed and proposed that the criterion should be whether:
“on the priority date of the patent, it would have been obvious to a person skilled in the art that the active ingredient in question was specifically and precisely identifiable in the wording of the claims of the basic patent. In the case of a combination of active ingredients, each active ingredient in that combination must be specifically, precisely and individually identifiable in the wording of the claims of the basic patent.”
The CJEU has not gone with either of these proposals, but has instead set out a test which is a more nuanced version of the AG’s test and which I think, for the first time, is tolerably clear and can reasonably be applied to future cases.
The ruling states:
Article 3(a) of Regulation No 469/2009 of the European Parliament and of the Council of 6 May 2009, concerning the supplementary protection certificate for medicinal products, must be interpreted as meaning that a product composed of several active ingredients with a combined effect is ‘protected by a basic patent in force’ within the meaning of that provision where, even if the combination of active ingredients of which that product is composed is not expressly mentioned in the claims of the basic patent, those claims relate necessarily and specifically to that combination. For that purpose, from the point of view of a person skilled in the art and on the basis of the prior art at the filing date or priority date of the basic patent:
– the combination of those active ingredients must necessarily, in the light of the description and drawings of that patent, fall under the invention covered by that patent, and
– each of those active ingredients must be specifically identifiable, in the light of all the information disclosed by that patent.
While emphasising that this is a matter for the referring Court, the CJEU makes clear how the test should be applied to the facts of the underlying case:
In the present case it is apparent, first, from the information in the order for reference that the description of the basic patent at issue contains no information as to the possibility that the invention covered by that patent could relate specifically to a combined effect of TD and emtricitabine for the purposes of the treatment of HIV. Consequently, it does not seem possible that a person skilled in the art, on the basis of the prior art at the filing date or priority date of that patent, would be able to understand how emtricitabine, in combination with TD, necessarily falls under the invention covered by that patent.
Like theologians poring over the latest papal encyclical, SPC enthusiasts have leapt on this judgment to try and work out what it really means. Unlike the gnomic utterances in Medeva (“specified in the wording of the claims of the basic patent”) and Lilly v HGS (“the claims relate, implicitly but necessarily and specifically, to the active ingredient in question”), some clarity and method is beginning to emerge.
What the CJEU is saying is that the active ingredient, or in the case of a combination product, each active ingredient in the combination, must specifically be indicated in the patent as being part of the invention. And by the “invention”, is not meant any consideration of the “clever bit” or inventive advance (as suggested by Arnold J) – the CJEU is relying only on the law that relates to the extent of protection and extent of invention – Article 69 EPC and Section 125 of the Patents Act. According to s125, the invention is “that specified in a claim of the specification of the application or patent, as the case may be, as interpreted by the description and any drawings contained in that specification”, and it is precisely that which the CJEU is referring to. The CJEU correctly rejected the AG’s opinion that focused on the claims alone, because Art 69 and s125 include the description and drawings as being part of the interpretative means. But the CJEU also said that the prior art can be taken into consideration, and it seems this would be relevant if the particular active ingredient in question was referred to in the patent indirectly in a manner that would lead the skilled person, in the light of the prior art, to the specific active ingredient.
By “specifically”, the CJEU means that the particular active ingredient must be somewhere indicated, and not simply a generic description that did not lead to the specific compound. The indication might be in the claims, in the description, or possibly in the prior art if, for example, the compound was sufficiently well known. How specific is needed is still a little unclear – obviously “other therapeutic ingredient” as in the current case is not enough (at least if the compound was not already known, but probably in any case). I would expect that a broad functional term would also not be enough. A therapeutic class might suffice, but I would expect that this might depend on how broad the class was, and how well known the particular active ingredient was within it. Hopefully two further CJEU cases will clarify this – in both the question is whether the term in the claim is specific enough to support an SPC. In the case of Sandoz v Searle, the claim specifies a broad structural formula, but not the exact combination of substituents leading to the specific compound, which is not disclosed in individualised form in the patent. In the Sitagliptin case, the term in the claims is a functional term. Good judgments in those cases would serve to clarify the situation a great deal.
So will patent attorneys now start adding lists of known drugs into patent applications for new chemical entities? And would this be effective to support a combination SPC? We may well see this happen, but I expect that if a whole pharmacopoeia of compounds is mentioned in the specification, the CJEU may ultimately rule that none of them is specifically disclosed as being part of the invention.
For the avoidance of doubt, I disagree with all of the jurisprudence on this topic. My preference would be a simple infringement test – if the active ingredient, combination or otherwise, would infringe the patent, then it should satisfy Art 3(a). But that ship has long sailed, and even the Swiss courts have now moved away from the infringement test. It seems that the best we can hope for now is clarity on the test that the CJEU intend to be used.Category Chemistry, IP (Intellectual Property) | Tags: CJEU, Combination Product, SPC
-
Legislation published for UK to join The Hague Agreement – and why did we not know who the IP Minister is?
1January 24, 2018 by IPAlchemist
I wrote yesterday that I tend to blog when I have a thought that I want to get out of my mind. Well, today I have two thoughts.
First, The Designs (International Registration of Industrial Designs) Order 2018 (S.I 2018 No. 23) has just been published (thanks to Peter Groves @IPso_Jure for tweeting this). This is the final legislation that is required for the UK to join the Geneva Act of the Hague Agreement, which is an international system for the registration of designs (similar to the Madrid system for trade marks). The UK was already effectively a member by virtue of the EU’s membership, and so UK entities could already file applications under the Hague system – it is a “closed system” so only entities based in member jurisdictions can use it. However, membership via the EU only allowed a Community Design to be designated, not a UK national design. Shortly, it will be possible to designate a UK national design as well as, or instead of, a Community Design.
The legal power for this legislation to be passed, and for the UK to sign up individually to the international design registration system, derives from section 8(1) of the Intellectual Property Act 2014 , and it is a bit of a shame that the Government has taken so long to enact the necessary Statutory Instrument. Given the uncertainty about the scope of Community Designs in the event of Brexit, the ability to designate a UK national right in addition to an EU right will be welcomed by many users of the system.
The UK now needs to deposit its instrument of ratification, which I suppose will happen quite quickly, and then (under Article 28(3) of the Geneva Act of the Hague Agreement) three months later the UK will become a member of the system.
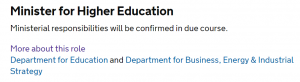
My second thought is that the legislation is dated 11 January 2018 and signed by Sam Gyimah. Now, I have been moaning (mostly on Twitter) since the government reshuffle on 8/9 January about the lack of clarity over who would be the next Minister for Intellectual Property (a position previously taken by Jo Johnson). Until today, the .gov.uk website described Sam Gyimah as “Minister for Higher Education” (only) and stated “Ministerial responsibilities will be confirmed in due course” – see the screenshots below. Today, this has changed to “Minister of State for Universities, Science, Research and Innovation” and intellectual property has been specifically listed as one of the responsibilities. (Thanks to @ipfederation for bringing this to my attention)
It is bad enough that it should take two weeks to set out where the ministerial responsibility for intellectual property lies. But the fact that this legislation was signed by Sam Gyimah on 11 January shows that he has in fact occupied this position the whole time. It also seems that those who attended the Alliance for IP reception on 17 January were told of the appointment. Why on earth should it take a further week to announce it properly?
Category IP (Intellectual Property), Political | Tags: Hague Agreement, Registered designs
-
The LGBT+ STEMinar 2018 – the conference for the future
1January 23, 2018 by IPAlchemist
I have been wanting to write a post about the LGBT+ STEMinar, but I was not really sure what I wanted to say. Having a thought in my head that I want to get out is frequently my driver for blogging, and when I don’t have it, things tend to get a bit delayed. In the meantime, others have written their accounts – you can see this on the website of the Royal Society of Chemistry (one of the several learned societies who sponsored the event) and this from my hero David Smith, a chemistry professor at the University of York.
It was one of the most tweeted conferences that I have come across, and the hashtag #LGBTSTEMinar18 will take you to a multitude of tweets and pictures. We were a trending hashtag on the day, and even attracted the attentions of a number of trolls, which most of us took as an affirmation of the significance of the event.
I have just come back from the annual general meeting of IP Inclusive, and this has finally spurred me to express a few thoughts about why the LGBT+ STEMinar is such an important event for me, and, from what I can tell from talking to other attendees, for many other people as well.
Firstly, although “interdisciplinary” is much talked about nowadays, it is extremely rare to attend an event that truly covers a whole range of disciplines within science. The programme is at the link here, and you can see that just in the talks there is chemistry, microscopy, astrophysics, biology, atmospheric physics and more. The posters were yet more diverse still. It is so rare to see scientists actively engaged outside their own discipline, and just sharing their enthusiasm for what interests them.
Secondly, the atmosphere at the conference was truly wonderful – people were respectful and engaged. The applause at the end of each talk had a quality not just of “thank you for finishing” but “really, thank you for sharing that with us”. There were no questions designed to show off the superior knowledge of the questioner, and people were respectful of their time allocations. And the socialising the evenings before and after the conference itself were great opportunities to chat to a whole range of fascinating scientists.
Thirdly, both the keynote speakers Beth Montague-Hellen at the beginning and Tom Welton at the end reminded me of just how far rights for LGBT+ people in the UK have come on in rather a short period of time of just a few decades. Being at the upper end of the age range of people attending, I am particularly conscious of this – not only was consensual sex between men decriminalised only the year before I was born, but also, when I came out at the age of 19, sex between men was still illegal for men of my age. This reminder is on the one hand very heartening, but on the other very concerning – what has been given in a short time can be taken away in a short time as well, and we cannot afford to be complacent. Many of these advances have taken place in only a few countries, and in much of the world LGBT+ people do not enjoy the freedoms that we do in the UK. And in the UK, even with the great progress that has been made, there is still much work to be done to promote LGBT+ visibility and inclusion both within the workplace and in wider society. It saddened me how many people present, a generation younger than me, spoke of less than full acceptance from their families and peers.
So it was also handy that we had a number of workshops on public engagement (that because of time pressures had to be run in parallel), exploring what we can all do to counter prejudice, and to increase visibility.
This year, my second to attend the conference, I was delighted to have my talk proposal accepted. I was very nervous, because I was aware of how few slots there were for speakers and how many excellent proposals had not been accepted. I spoke about the patent litigation on pregabalin, and the effect on the drug reimbursement price – truly interdisciplinary even by the standards of this most diverse event. I hope that I succeeded in my aim of communicating the fascination I have with how complex litigation unfolds and affects those involved, not just the parties to the litigation.
I also took along a poster about IP Out, and several people came over to talk to me about patents and about the work that IP Out is doing.
On a more personal note, it was lovely to have the event in York – every time I go there I am struck afresh by quite what a beautiful place it is. But I grew up in Yorkshire, and I cannot say that my memories are entirely happy, as it was not a forgiving environment for a boy who didn’t entirely fit in. So it is always with mixed emotions that I journey from London up the East Coast line. The transportation was also not kind to us, as there were train delays both the day before and on the day of the conference. Happily, though, I still made it.
In my title I called this the “conference for the future”, as I think that the event gave the participants the space to be authentically ourselves. We would like our environments, and especially our workplaces, to be like this all the time. In the meantime, for just one day a year, we have this. So I look forward to seeing everyone next year, which will be hosted by the Royal Astronomical Society and the Institute of Physics in London.
Category Chemistry, IP (Intellectual Property), Patents, Science | Tags: LGBTSTEMinar18
-
Why were the SPCs for Pregabalin allowed to lapse? I have a theory…
1January 19, 2018 by IPAlchemist
The patent litigation on pregabalin has been one of the most important UK patent stories of this century. It has raised important questions about the scope and meaning of second medical use patents, and has led to entirely new forms of court order (the Patents Court ordering NHS England to issue instructions on the prescribing of pregabalin by brand rather than generically). At the time, I wrote about the story extensively on the IPKat blog. (My last post is here; my posts on the main first instance decisions are here and here; links to earlier decisions are here.) I have been looking at the situation again because I am collaborating with Ben Goldacre and Richard Croker at the Department of Primary Care Health Sciences, University of Oxford on a study of the effect of the litigation on pregabalin prescribing and cost.
There was one recurring fact in the litigation that was often referred to but never explained. The Supplementary Protection Certificate (SPC/GB04/034) based on the original product patent for pregabalin (EP0641330) was applied for in October 2004, following the granting of the European marketing authorisation to Pfizer for their Lyrica brand pregabalin, and granted in February 2005. But then in May 2013 the renewal fees required to bring it into force upon expiry of EP0641330 were not paid, so that it lapsed. It is a vanishingly rare occurrence to allow an SPC to lapse in this manner, and it must have been intentional. The question is, why would Pfizer do this? Incidentally, the owner of the patent and SPC is not Pfizer, but Northwestern University. There is no licence recorded on the UK IPO register, but licences are often not recorded and I presume that Pfizer (or one of its subsidiaries) is actually a licensee under the patent.
While looking into the case, I noticed that the US application from which EP0641330 claims priority is a continuation-in-part of an earlier US application. This is often a signpost to a possible self-collision issue, and so I took a closer look.
EP0641330 is based on international patent application WO93/23383, and claims priority from US application number 07/886,080. (“Claiming priority” is a system where you can file an application up to a year later than the earlier application, but it is regarded for the purposes of considering patentability [novelty and inventive step] as having been filed at the earlier date. Such a priority claim is valid only if the subject matter of the claim in question is present in both applications, and also provided that the earlier of the two applications is the first application for that subject matter). However, 07/886,080 is a continuation-in-part of US application 07/618,692, and a PCT application WO92/09560 was also filed claiming priority from that earlier US application.
The timeline is as follows:
27 November 1990 US 07/618,692 filed
20 November 1991 WO92/09560 filed
20 May 1992 US 07/886,080 filed
11 June 1992 WO92/09560 published
18 May 1993 WO93/23383 filedNow, if we consider the right to priority of the claim to pregabalin in EP0641330, the corresponding subject matter is present in 07/886,080 and therefore on the face of it the first part of the test for a valid priority claim is satisfied – the subject matter is present in both applications. On that basis, WO92/09560 is not available as prior art, because it was published after 20 May 1992. (If this were not the case, I would expect the EPO examiner to have noticed it).
The difference between WO92/09560 and EP0641330 is that in EP0641330 pregabalin is claimed as a single enantiomer – the S-(+) enantiomer. In WO92/09560, by contrast, while the pregabalin molecule is disclosed, it is only made and tested as the racemate. However, WO92/09560 also states:
“The compounds made in accordance with the present invention can contain one or several asymmetric carbon atoms. The invention includes the individual diastereomers or enantiomers, and the mixtures thereof. The individual diastereomers or enantiomers may be prepared or isolated by methods already well known in the art.”
There is a plausible argument that this passage, in combination with the disclosure of the racemic pregabalin, is sufficient to count as a disclosure of the S-(+) enantiomer. In that case, however, WO92/09560 (or its priority application 07/618,692) is the “first” application for that subject matter, and not 07/886,080. That would make the priority claim invalid so that the effective date for considering the patentability of EP0641330 would become the filing date of WO93/23383, namely 18 May 1993. Thus WO92/09560 would become novelty-destroying prior art, because it was published before the filing date of WO93/23383 and thus EP0641330.
Now, all of this is arguable both ways, and it is possible that a court would not accept the argument. It is also a fairly subtle point, and I can imagine an EPO examiner missing it. It does not surprise me that the patent was nevertheless granted – on the face of it if the priority claim of WO93/23383 is valid, then WO92/09560 is not prior art at all since it was never filed at the European Patent Office as a European application. (If WO92/09560 has been filed as a European application, it would have been considered by the EPO examiner as “novelty-only” prior art under Article 54(3) EPC as being filed before, but published after, the priority date of WO93/23383). It is only if the additional criterion, whether 07/886,080 is the “first application” in respect of the subject matter of the S-(+) enantiomer of pregabalin, is considered that a potential problem becomes apparent.
None of this would apply in the USA, where the rules about your own earlier applications counting as prior art against your own application are completely different from the rules in Europe.
Also, a possible reason why the patent might be invalid is not of itself a reason for Pfizer to drop the SPC. Pharmaceutical patents are often found to be invalid by national courts, and although obviously the proprietor then loses the patent case, there is usually no further adverse outcome.
However, in 2009 the EU Commission published the Final Report in its competition inquiry into the pharmaceutical sector, which raised a number of questions about behaviours that had previously been considered unproblematic. Then in July 2010 the General Court upheld a finding of abuse of dominant position against AstraZeneca in relation to Losec (omeprazole), for actions including providing incorrect information about the date of the first marketing authorisation when applying for supplementary protection certificates, and this was upheld by the Court of Justice of the European Union in December 2012.
Moreover, in January 2012, the Italian Competition Authority sanctioned Pfizer for abuse of a dominant position relating to latanoprost consisting of various aspects including basing an SPC on a divisional application. This was quashed by the Regional Administrative Court of Lazio in September 2012, but then reinstated in February 2014 by the Consiglio di Stato.
All of these events together created a perception around 2010-2012 that, at least in respect of an SPC (if not necessarily in respect of a patent), the proprietor might (under competition law if not under any intellectual property law) be under a kind of good faith obligation that was more extensive than had previously been the case in Europe, and that the EU competition authorities might regard breach of such a good faith requirement as constituting an abuse of a dominant position. That perception is perhaps less prominent now, but at the time such views were widely discussed. A person might therefore have had a concern that a patent proprietor applying for an SPC based on a patent that the proprietor knew or should have known to be invalid, because the reason for invalidity was their own prior art, might be found to be an abuse of a dominant position under competition law.
There is no clear authority that it is an abuse of a dominant position to apply for an SPC based on an invalid patent (even where the patentee should know it is invalid, for example because the prior art is their own related application), but it may well have seemed in 2013 that this was the direction in which competition law was heading. The EU Commission can fine an undertaking up to 10% of worldwide turnover, so the risks involved if competition law comes into play are very high.
By not bringing the SPC into force, it was pretty much guaranteed that there would never be any litigation under the patent, since it would expire before the end of the data exclusivity period. On the other hand, if the SPC were brought into force, there would inevitably be litigation with generic companies, and there would be a risk that the patent might be found invalid for this self-collision reason. Once that reason became public by reason of the litigation, there could then arise a concern that this would lead to EU Commission action under competition law.
I do not know why Pfizer decided not to bring the SPC into force. However, this priority issue leading to a potential self-collision provides a plausible reason (and I have not seen any other possible reason suggested) why someone might have decided that it was too risky to bring the SPC into force. I wonder whether anyone can tell me whether it is anywhere close to the real reason.
Category Chemistry, IP (Intellectual Property), Patents, Science | Tags: patents, pregabalin, supplementary protection certificate
-
Report of the case management hearing on 19 July 2016 of the legal challenges to Brexit
0July 25, 2016 by IPAlchemist
The IP Alchemist and a couple of his colleagues from EIP went to Royal Courts of Justice on 19 July to watch the hearing to give directions and timetable to the various legal challenges to how, following the 23 June Referendum, the Government may take a decision to leave the EU and notify this decision to the EU under Article 50 of the Treaty on European Union.
The hearing was before Sir Brain Leveson, President of the Queen’s Bench Division, and Mr Justice Cranston, from 10am to around 11.30am. Listed for Court 3, it was moved at the last minute to Court 4 because of pressure of numbers attending (both for the parties and in the public benches).
Claimants:
There were four main cases in existence or contemplation: Dos Santos (generally referred to by the name of the claimant), and Mishcon de Reya, Bindmans, and Croft (generally referred to by the names of the respective instructing solicitors). The Dos Santos case was the only one where a claim form had actually been issued and they wished to be the lead case (or at least joint lead case), but there was an issue of them not complying with the pre-action protocols (being the only reason why their case was ostensibly further advanced), and they were also seeking a protective costs order, which the Mishcon claimants were not. Dos Santos mentioned they would potentially withdraw their request for a protective costs order in order to remove this stumbling block to their desire to be the lead case. Despite this, it was decided that the Mishcon de Reya case, represented by Lord Pannick QC, would be the lead case. It was agreed by the Court that correspondence should have the litigants’ names redacted in order to avoid a continuation of the abusive and threatening communications that Mishcon and their clients have experienced as a result of this case (which had been notified to the Court by a letter from Lord Pannick QC). Sir Brian Leveson indicated that the Court took these incidents very seriously, and would be giving consideration to whether they may amount to contempt of court.All of the other cases were to be stayed, but permission would be granted for them to intervene in the main case. Dominic Chambers QC for Dos Santos appeared to indicate that, if not the lead claimant, his client would prefer not to intervene but to be heard as an interested party.
The Bindmans case was organised and crowdfunded by Jolyon Maugham QC of Devereux Chambers – he was present at the hearing but not acting for any party (he tweeted for part of the hearing but had to leave to give a lecture). Information about the case can be read on his blog here:
https://waitingfortax.com/2016/07/08/article-50-our-letter-to-the-government/
The Croft case is apparently on behalf of some UK citizens resident in France, presumably challenging the possible loss to them of EU residence entitlement.
In addition there were two litigants in person, not present at the hearing, Hardy and Jacobson. At least one of these may be wishing to challenge the constitutionality of the referendum, in addition to the constitutional requirements for decision to leave the EU under Article 50.
In view of the large number of claimants and potential claimants, it was agreed to use group email as the method of correspondence. Concern was expressed that email correspondence could lead to leaks and therefore potential further abuse. Sir Brian Leveson made clear that he expected confidentiality to be observed and would take a dim view of any of the material surfacing on any blogs.
The defendant, originally assumed at least by the Dos Santos team to be the Chancellor of the Duchy of Lancaster, should in fact be the Secretary of State for Exiting the European Union (“Minister for Brexit”). The defendant was represented by Jason Coppel QC.
Jason Coppel QC for the Government confirmed that the Government does not intend to make a notification under Article 50 before the end of the year. Accordingly, the hearing was set for mid-October. While not (as far as we understood) making a formal order, Sir Brian Leveson made clear that the Court expected to be informed if the Government changes its plans on timing. It appears to be assumed that there will be a leapfrog appeal, as Sir Brian Leveson believes this case meets the criteria to go straight to the Supreme Court, and no party challenged this. Sir Brian Leveson stated that the Supreme Court would be contacted immediately in order to ensure that there was space in the diary. The case will be heard by the Lord Chief Justice, but the other two judges are yet to be confirmed.
Timetable:
July 25th – deadline for Government to respond to the pre-action letters (so far the Government had not responded to any of the claimants, and Mr Coppel was unable to indicate whether any progress had been made on the responses yet)
July 29th- Mishcon de Reya claim to be issued
September 2nd- detailed response required from defendant
September 14th- skeleton arguments from claimant must be submitted
September 21st- deadline for submissions from interveners and interested parties (only if additional to points made by claimant)
September 30th- skeleton arguments from defendant must be submitted
October 17th- trial to be held for 2-3 days
If the hearing needs to be moved, for example if Article 50 to be activated earlier (or later) than currently planned, then other dates can be moved accordingly.
Sir Brian Leveson indicated [paraphrasing somewhat but more or less in his words]: You should have no doubt that the Court takes this case extremely seriously and will act expeditiously for its disposal; the Lord Chief Justice will require concision and expedition; and, concerning the timetable, there will be liberty to apply for variation but “not by much”, and case will “continue to be managed over the summer”.
Good sources of information about these cases are the Jack of Kent blog (see for example here) and its author David Green’s Twitter @DavidAllenGreen; and also on Twitter Joshua Rosenberg @JoshuaRozenberg. Other reports of this hearing are in The Guardian here and Full Fact here.
Category IP (Intellectual Property), Political | Tags:
-
RSC Profile
3April 4, 2013 by IPAlchemist
Over the last year or so, I have been involved in a number of projects related to the public perception and understanding of chemistry, and also showing to current or aspiring chemists what possible careers are available for them, and what chemistry-related jobs might look like.
On Twitter, we have had #RealTimeChem (see @RealTimeChem, organised by @DrGalactic, whose blog is on my blogroll). The next event is going to be a week, not just a day, beginning on 22 April, so do all look out for that. I shall be at BIO in Chicago that week, so I am hoping to tweet and blog from there. It is my first time attending the BIO convention, so I am very excited about it.
The other week I was also thrilled to be added to @JessTheChemist ‘s family tree of tweeting chemists (where everyone is connected via a current or former supervisor). Her blog is on my blogroll, and the post with the family tree is here.
On the blogosphere, See Arr Oh hosted a Chem Coach Carnival on his blog Just Like Cooking, also on my Blogroll, last October, which I participated in here.
In a perhaps similar vein, the Royal Society of Chemistry has every month in RSC News, which accompanies Chemistry World, the magazine for RSC members, a profile of a chemist. And I am in the April edition, which you can find here (the profile is on page 7) or on the RSC Blog The Reaction here.
As it happens, there has been a bit of an intellectual property theme going on in the RSC News profiles recently, because just a few months ago the towering IP barrister Michael Edenborough QC was likewise featured – you can see his profile here. I actually only found out quite recently that his background was quite so strongly chemical – barristers practise in a wider range of technical and legal fields than patent attorneys so have a diverse array of backgrounds.
I hope that this little array may help any chemists out there who are considering what direction their career may next take.
Category Chemistry, IP (Intellectual Property) | Tags: Careers, Chem Coach Carnival, Chemistry, RealTimeChem, RSC
-
Factum est silentium…
0March 18, 2013 by IPAlchemist
…or The Sound of Silence
I have not explained, dear readers, why I have been out of commission for such a long time. I did not inform you, as I should have done, that I re-joined the IPKat weblog as a permanent member earlier in the year. So such blogging activity as I have undertaken has been there. I won’t post here when I post things to the IPKat, but I will from time to time update “Publications” with my IPKat pieces.
I do have a couple of non-IP related things to post about, so do not give up hope here. I shall still be blogging from time to time. I won’t spoil the surprise by saying what they are.
Category IP (Intellectual Property), News | Tags: Update
-
Genesis 2012 – Better late than never?
1January 18, 2013 by IPAlchemist
It is now over a month since Genesis, the UK’s flagship life science and healthcare networking conference. I had always intended blogging about it – I was very excited to attend the whole event for the first time, because in previous years the most that I managed was to pop in for a little while, or attend the dinner. The problem is that, after the event was over, nothing really came into my mind that I wanted to say. So the blog piece got put off, until now, at the one-month stage, I feel I really need to write it, whatever.
Normally when I go to an event, I come away with something that I want to say, but on this occasion it didn’t really happen. It is not that I did not enjoy the event – I enjoyed it immensely. I met many interesting people for the first time, as well as running into various One Nucleus stalwarts that it was a pleasure to see again. There were of course many patent attorneys in attendance (although with some notable and noticeable absences), and it is rarely disappointing to meet a patent attorney. There were many interesting and stimulating discussions, as well as the formal presentations.
In particular I attended the afternoon session on Antibody-Based Therapeutics which yielded many fascinating brief stories (although one, which I feel I should not name, was basically “We have great idea but it is so early stage we can’t tell you what it is yet. It might not work – we don’t know yet, but if it does it will be amazing”).
In the morning I attended a case study on the deal between Astex, Cancer Research Technology and The Institute of Cancer Research relating, of course, to an anticancer compound. This revealed fascinating insights into how such complex deals come into existence and what drives the terms and the choice of partner.
I also attended the morning plenary session and the afternoon plenary debate. And perhaps that is the issue. Annual events such as Genesis prompt a certain amount of navel gazing. The industry as a whole, in its widest sense including service providers and academia as well as pharmaceutical and biotech companies of whatever type, considers “are we in good shape”? And now seems a particularly troubling time to ask this question, because, as the plenary debate made clear, one can equally argue for optimism pointing to all sorts of wonderful positive signs, as for pessimism pointing to all the harbingers of doom. And I wonder whether it might not be better if the answer was clearly negative, because then we could agree that there is a problem and do something about it: I feel maybe that the lack of consensus is itself the reason for the feeling of unease.
I will end on a harbinger of optimism, a fellow blogger that I have added to my blogroll, Lucy Robertshaw. Lucy was a model of optimism and enterprise, having moved from the UK to Sweden to set up her own consultancy company. She also cleverly worked out that if you get the right photo, you can do quite a short post! That was my APAA strategy, but foolishly I took no snaps at Genesis.
Category Chemistry, IP (Intellectual Property), News | Tags: Genesis, healthcare, life science, One-Nucleus
-
APAA – A better photographer
0November 11, 2012 by IPAlchemist
On the excursion day at APAA, I was accompanied by the excellent Mr Shinji Okayama, a Japanese patent attorney who has taken the courageous decision to move from his native Japan to Denmark to work at Plougmann & Vingtoft. I had major camera envy, and the results testify to both his superior equipment and his superior skill. Shinji has kindly sent me some pictures, and permitted me to reproduce them on my blog, so here you are. Thank you Shinji.
Category IP (Intellectual Property), Travel | Tags: APAA
-
Chiang Mai APAA 2012 – Day 4 and end of Congress
0November 1, 2012 by IPAlchemist
It is now Thursday afternoon, and my last few posts have all been pictures, so I think it is time to return to my familiar (and preferred) medium of language.
My photos were not that great, some taken just with iphone with challenging illumination conditions. They then needed downloading onto my laptop, followed by an attempt to indentify ones that even merited posting. Then there was quite a time-consuming and error-prone uploading process because of the rather limited bandwidth of the WiFi connection here. So apologies for the lack of real-time illustrations.
The excursion day was lovely, as I hope the piccies show. I had great travelling companions, even if our guide was a little shrill and over-enthusiastic. The elephant centre was much more fun than I had imagined, and I was quite surprised to find myself really looking forward to the elephant ride. I don’t know how humane it really is, but it was a wonderful experience.
The temple is apparently the oldest in northern Thailand, and was very moving. The main hall was being renovated, but the smaller halls were still interesting, and the main central pagoda, of copper (it seemed) with gold overlay, was magnificent – this is what is in the picture I posted.
Mercifully, I then had time for a shower before heading to a lovely reception hosted by the pan-Asian firm of Axis. We were treated to some wonderful hospitality.
So what then of the title of this post, the final day?
Well, firstly it was the only day where I went to any business session of the conference (I don’t count the opening ceremony). Most of the delegates in fact largely disregard the official programme, and indeed significant portions are reserved for members of APAA, or sometimes even only for council members. I was struck by how, in contrast to the open informality of the rest of the proceedings, the formal programme was very – well – formal.
I luckily joined the session in time to learn that the special topic of the Design standing committee for next year will be mobile communication devices. As I am chairing a session on this very topic at a conference to be held by and at OHIM next April (to celebrate 10 years of the Community Registered Design) I was very interested to see this. I hope that we can collaborate and compare notes.
During the rest of the day I had some scheduled and unscheduled meetings, and was, for this one day only, also able to find some time during the day to catch up with some work from back home.
Then it was off to the Royal Park Rajapruek for the closing banquet. This had apparently been intended to held al fresco, but torrential rain earlier in the day had scuppered this plan. So we were divided into two halls, one for those who had pre-reserved tables, and one for the rest of us. Our hall had the disadvantage of hearing the fantastic live music only via relay, with the concomitant advantage of being completely unable to hear, and therefore able cheerfully to ignore, the speeches.
On entering, there was a parade of ladies dressed in the national costumes of, it seemed, all of the APAA recognized countries, which was quite a sight. Indeed delegates were invited to wear national dress, and, while most like me stuck to suit, many people were wearing the most fabulous and stunning outfits. I elected to interpose myself next to the Japanese-dressed lady for the entry photo.
The meal was completely western, the only time so far that this happened, but, like all the other food during the conference, completely delicious.
By the end of all of this, I was utterly done in. So even though I really wanted to get to the hospitality suite, which I had not really experienced properly the whole conference (thereby missing the legendary and apparently inimitable APAA band), I elected to stay on the bus and go back to my hotel.
You might have thought that this was the end. But not a bit of it. The firm of Tilleke & Gibbins decided that we had not yet had enough of networking and stuffing our faces, and so they kindly organized an after-event brunch on the Thursday morning. This was a truly delightful affair, much more relaxed in the way that only an after-event can be, which gave us a chance to re-connect with some of those who we had met over the previous four days, and even meet a few additional people. Moreover, significant numbers of people were actually NOT WEARING SUITS! In astonishing contrast to the first day, where the “smart casual” dress code was interpreted by most delegates and meaning “suit and tie”. The setting was also stunning, while the food and drink was fabulous (as, to be fair, it had been all through the conference). I would like to thank Tilleke & Gibbins for a great closing event.
So now it really is the end of #APAA2012, and my holiday can begin. Or, if you like, we can turn our thoughts to Hanoi and #APAA2013!
Category IP (Intellectual Property), Travel | Tags: APAA