-
IP Out Event – January Drinks
1January 31, 2018 by IPAlchemist
I really did not think I would be writing an event report of an event that had no speakers, but actually quite a lot happened at the IP Out drinks last night, and so I wanted to record a few thoughts.
It’s been less than 18 months since our first event, and we are already on our fifth. Feedback from attendees from questionnaires at our previous events continued to show support for the idea of a “social only” event, without any speakers or particular topic. So this time, for the first time, we held an event in a bar, rather than in the offices of a law firm, where all our previous events have taken place. We went for the New Bloomsbury Set, who gave us our own area and treated us very well. Since the organisation required for social drinks is much lower than for a speaker event, I am wondering whether we could make this a regular event on a fixed timetable. If we had it say every month, for example, would people want to come at that frequency?
Numbers were lower than at the more formal meetings, but we still had a decent turnout, and my impression is that a drinks event is not overall less popular, but rather that fewer people who identify as allies come if there is not a specific topic under discussion. That’s not a problem at all, but I do want to reiterate that allies are welcome at all IP Out events. I did worry that people would be deterred from attending an event where they had to buy drinks for themselves and each other rather than refreshments being kindly supplied by our host firm (and our host firms have always been very generous), but from what I saw that did not cause a problem at all.
Gratifyingly, from my rough count, about a quarter of people attending were at an IP Out event for the first time, so we are still succeeding in reaching out to new people, while remaining well-supported by our excellent committee and regular participants. I was also delighted to see that we continue to attract a wide cross-section of people from across the intellectual property law and related professions, which, as I discovered at the IP Inclusive AGM, is an aspect in which the IP Out group is really excelling. But it seems that we cannot say too often, so I am saying it again – IP Out is not only for patent attorneys, trade mark attorneys, solicitors, barristers, notaries, legal executives, members of the judiciary, trainees for those roles, and members of the judiciary, but is for anyone working in IP, regardless of your role, which, in a non-exclusive list, could include secretarial, formalities, records, paralegal, searcher, journalist, translator, patent illustrator, conference organiser, HR, IT, accounts, marketing, or office services. If you are in a patent and trade mark attorney firm, an IP law firm, the IP department of an IP law firm, an in-house IP department, legal publisher, search firm, translation firm, patent illustration firm, barristers’ chambers, or similar or related organisation that I can’t think of at the moment, we would love to have you join us for any event that you wanted to attend. They are all listed on our webpage here.
Thank you again to everyone who attended last night and contributing to such a splendid evening.
Category IP Out | Tags: IP Out drinks
-
Legislation published for UK to join The Hague Agreement – and why did we not know who the IP Minister is?
1January 24, 2018 by IPAlchemist
I wrote yesterday that I tend to blog when I have a thought that I want to get out of my mind. Well, today I have two thoughts.
First, The Designs (International Registration of Industrial Designs) Order 2018 (S.I 2018 No. 23) has just been published (thanks to Peter Groves @IPso_Jure for tweeting this). This is the final legislation that is required for the UK to join the Geneva Act of the Hague Agreement, which is an international system for the registration of designs (similar to the Madrid system for trade marks). The UK was already effectively a member by virtue of the EU’s membership, and so UK entities could already file applications under the Hague system – it is a “closed system” so only entities based in member jurisdictions can use it. However, membership via the EU only allowed a Community Design to be designated, not a UK national design. Shortly, it will be possible to designate a UK national design as well as, or instead of, a Community Design.
The legal power for this legislation to be passed, and for the UK to sign up individually to the international design registration system, derives from section 8(1) of the Intellectual Property Act 2014 , and it is a bit of a shame that the Government has taken so long to enact the necessary Statutory Instrument. Given the uncertainty about the scope of Community Designs in the event of Brexit, the ability to designate a UK national right in addition to an EU right will be welcomed by many users of the system.
The UK now needs to deposit its instrument of ratification, which I suppose will happen quite quickly, and then (under Article 28(3) of the Geneva Act of the Hague Agreement) three months later the UK will become a member of the system.
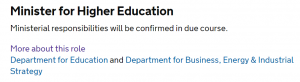
My second thought is that the legislation is dated 11 January 2018 and signed by Sam Gyimah. Now, I have been moaning (mostly on Twitter) since the government reshuffle on 8/9 January about the lack of clarity over who would be the next Minister for Intellectual Property (a position previously taken by Jo Johnson). Until today, the .gov.uk website described Sam Gyimah as “Minister for Higher Education” (only) and stated “Ministerial responsibilities will be confirmed in due course” – see the screenshots below. Today, this has changed to “Minister of State for Universities, Science, Research and Innovation” and intellectual property has been specifically listed as one of the responsibilities. (Thanks to @ipfederation for bringing this to my attention)
It is bad enough that it should take two weeks to set out where the ministerial responsibility for intellectual property lies. But the fact that this legislation was signed by Sam Gyimah on 11 January shows that he has in fact occupied this position the whole time. It also seems that those who attended the Alliance for IP reception on 17 January were told of the appointment. Why on earth should it take a further week to announce it properly?
Category IP (Intellectual Property), Political | Tags: Hague Agreement, Registered designs
-
The LGBT+ STEMinar 2018 – the conference for the future
1January 23, 2018 by IPAlchemist
I have been wanting to write a post about the LGBT+ STEMinar, but I was not really sure what I wanted to say. Having a thought in my head that I want to get out is frequently my driver for blogging, and when I don’t have it, things tend to get a bit delayed. In the meantime, others have written their accounts – you can see this on the website of the Royal Society of Chemistry (one of the several learned societies who sponsored the event) and this from my hero David Smith, a chemistry professor at the University of York.
It was one of the most tweeted conferences that I have come across, and the hashtag #LGBTSTEMinar18 will take you to a multitude of tweets and pictures. We were a trending hashtag on the day, and even attracted the attentions of a number of trolls, which most of us took as an affirmation of the significance of the event.
I have just come back from the annual general meeting of IP Inclusive, and this has finally spurred me to express a few thoughts about why the LGBT+ STEMinar is such an important event for me, and, from what I can tell from talking to other attendees, for many other people as well.
Firstly, although “interdisciplinary” is much talked about nowadays, it is extremely rare to attend an event that truly covers a whole range of disciplines within science. The programme is at the link here, and you can see that just in the talks there is chemistry, microscopy, astrophysics, biology, atmospheric physics and more. The posters were yet more diverse still. It is so rare to see scientists actively engaged outside their own discipline, and just sharing their enthusiasm for what interests them.
Secondly, the atmosphere at the conference was truly wonderful – people were respectful and engaged. The applause at the end of each talk had a quality not just of “thank you for finishing” but “really, thank you for sharing that with us”. There were no questions designed to show off the superior knowledge of the questioner, and people were respectful of their time allocations. And the socialising the evenings before and after the conference itself were great opportunities to chat to a whole range of fascinating scientists.
Thirdly, both the keynote speakers Beth Montague-Hellen at the beginning and Tom Welton at the end reminded me of just how far rights for LGBT+ people in the UK have come on in rather a short period of time of just a few decades. Being at the upper end of the age range of people attending, I am particularly conscious of this – not only was consensual sex between men decriminalised only the year before I was born, but also, when I came out at the age of 19, sex between men was still illegal for men of my age. This reminder is on the one hand very heartening, but on the other very concerning – what has been given in a short time can be taken away in a short time as well, and we cannot afford to be complacent. Many of these advances have taken place in only a few countries, and in much of the world LGBT+ people do not enjoy the freedoms that we do in the UK. And in the UK, even with the great progress that has been made, there is still much work to be done to promote LGBT+ visibility and inclusion both within the workplace and in wider society. It saddened me how many people present, a generation younger than me, spoke of less than full acceptance from their families and peers.
So it was also handy that we had a number of workshops on public engagement (that because of time pressures had to be run in parallel), exploring what we can all do to counter prejudice, and to increase visibility.
This year, my second to attend the conference, I was delighted to have my talk proposal accepted. I was very nervous, because I was aware of how few slots there were for speakers and how many excellent proposals had not been accepted. I spoke about the patent litigation on pregabalin, and the effect on the drug reimbursement price – truly interdisciplinary even by the standards of this most diverse event. I hope that I succeeded in my aim of communicating the fascination I have with how complex litigation unfolds and affects those involved, not just the parties to the litigation.
I also took along a poster about IP Out, and several people came over to talk to me about patents and about the work that IP Out is doing.
On a more personal note, it was lovely to have the event in York – every time I go there I am struck afresh by quite what a beautiful place it is. But I grew up in Yorkshire, and I cannot say that my memories are entirely happy, as it was not a forgiving environment for a boy who didn’t entirely fit in. So it is always with mixed emotions that I journey from London up the East Coast line. The transportation was also not kind to us, as there were train delays both the day before and on the day of the conference. Happily, though, I still made it.
In my title I called this the “conference for the future”, as I think that the event gave the participants the space to be authentically ourselves. We would like our environments, and especially our workplaces, to be like this all the time. In the meantime, for just one day a year, we have this. So I look forward to seeing everyone next year, which will be hosted by the Royal Astronomical Society and the Institute of Physics in London.
Category Chemistry, IP (Intellectual Property), Patents, Science | Tags: LGBTSTEMinar18
-
Why were the SPCs for Pregabalin allowed to lapse? I have a theory…
1January 19, 2018 by IPAlchemist
The patent litigation on pregabalin has been one of the most important UK patent stories of this century. It has raised important questions about the scope and meaning of second medical use patents, and has led to entirely new forms of court order (the Patents Court ordering NHS England to issue instructions on the prescribing of pregabalin by brand rather than generically). At the time, I wrote about the story extensively on the IPKat blog. (My last post is here; my posts on the main first instance decisions are here and here; links to earlier decisions are here.) I have been looking at the situation again because I am collaborating with Ben Goldacre and Richard Croker at the Department of Primary Care Health Sciences, University of Oxford on a study of the effect of the litigation on pregabalin prescribing and cost.
There was one recurring fact in the litigation that was often referred to but never explained. The Supplementary Protection Certificate (SPC/GB04/034) based on the original product patent for pregabalin (EP0641330) was applied for in October 2004, following the granting of the European marketing authorisation to Pfizer for their Lyrica brand pregabalin, and granted in February 2005. But then in May 2013 the renewal fees required to bring it into force upon expiry of EP0641330 were not paid, so that it lapsed. It is a vanishingly rare occurrence to allow an SPC to lapse in this manner, and it must have been intentional. The question is, why would Pfizer do this? Incidentally, the owner of the patent and SPC is not Pfizer, but Northwestern University. There is no licence recorded on the UK IPO register, but licences are often not recorded and I presume that Pfizer (or one of its subsidiaries) is actually a licensee under the patent.
While looking into the case, I noticed that the US application from which EP0641330 claims priority is a continuation-in-part of an earlier US application. This is often a signpost to a possible self-collision issue, and so I took a closer look.
EP0641330 is based on international patent application WO93/23383, and claims priority from US application number 07/886,080. (“Claiming priority” is a system where you can file an application up to a year later than the earlier application, but it is regarded for the purposes of considering patentability [novelty and inventive step] as having been filed at the earlier date. Such a priority claim is valid only if the subject matter of the claim in question is present in both applications, and also provided that the earlier of the two applications is the first application for that subject matter). However, 07/886,080 is a continuation-in-part of US application 07/618,692, and a PCT application WO92/09560 was also filed claiming priority from that earlier US application.
The timeline is as follows:
27 November 1990 US 07/618,692 filed
20 November 1991 WO92/09560 filed
20 May 1992 US 07/886,080 filed
11 June 1992 WO92/09560 published
18 May 1993 WO93/23383 filedNow, if we consider the right to priority of the claim to pregabalin in EP0641330, the corresponding subject matter is present in 07/886,080 and therefore on the face of it the first part of the test for a valid priority claim is satisfied – the subject matter is present in both applications. On that basis, WO92/09560 is not available as prior art, because it was published after 20 May 1992. (If this were not the case, I would expect the EPO examiner to have noticed it).
The difference between WO92/09560 and EP0641330 is that in EP0641330 pregabalin is claimed as a single enantiomer – the S-(+) enantiomer. In WO92/09560, by contrast, while the pregabalin molecule is disclosed, it is only made and tested as the racemate. However, WO92/09560 also states:
“The compounds made in accordance with the present invention can contain one or several asymmetric carbon atoms. The invention includes the individual diastereomers or enantiomers, and the mixtures thereof. The individual diastereomers or enantiomers may be prepared or isolated by methods already well known in the art.”
There is a plausible argument that this passage, in combination with the disclosure of the racemic pregabalin, is sufficient to count as a disclosure of the S-(+) enantiomer. In that case, however, WO92/09560 (or its priority application 07/618,692) is the “first” application for that subject matter, and not 07/886,080. That would make the priority claim invalid so that the effective date for considering the patentability of EP0641330 would become the filing date of WO93/23383, namely 18 May 1993. Thus WO92/09560 would become novelty-destroying prior art, because it was published before the filing date of WO93/23383 and thus EP0641330.
Now, all of this is arguable both ways, and it is possible that a court would not accept the argument. It is also a fairly subtle point, and I can imagine an EPO examiner missing it. It does not surprise me that the patent was nevertheless granted – on the face of it if the priority claim of WO93/23383 is valid, then WO92/09560 is not prior art at all since it was never filed at the European Patent Office as a European application. (If WO92/09560 has been filed as a European application, it would have been considered by the EPO examiner as “novelty-only” prior art under Article 54(3) EPC as being filed before, but published after, the priority date of WO93/23383). It is only if the additional criterion, whether 07/886,080 is the “first application” in respect of the subject matter of the S-(+) enantiomer of pregabalin, is considered that a potential problem becomes apparent.
None of this would apply in the USA, where the rules about your own earlier applications counting as prior art against your own application are completely different from the rules in Europe.
Also, a possible reason why the patent might be invalid is not of itself a reason for Pfizer to drop the SPC. Pharmaceutical patents are often found to be invalid by national courts, and although obviously the proprietor then loses the patent case, there is usually no further adverse outcome.
However, in 2009 the EU Commission published the Final Report in its competition inquiry into the pharmaceutical sector, which raised a number of questions about behaviours that had previously been considered unproblematic. Then in July 2010 the General Court upheld a finding of abuse of dominant position against AstraZeneca in relation to Losec (omeprazole), for actions including providing incorrect information about the date of the first marketing authorisation when applying for supplementary protection certificates, and this was upheld by the Court of Justice of the European Union in December 2012.
Moreover, in January 2012, the Italian Competition Authority sanctioned Pfizer for abuse of a dominant position relating to latanoprost consisting of various aspects including basing an SPC on a divisional application. This was quashed by the Regional Administrative Court of Lazio in September 2012, but then reinstated in February 2014 by the Consiglio di Stato.
All of these events together created a perception around 2010-2012 that, at least in respect of an SPC (if not necessarily in respect of a patent), the proprietor might (under competition law if not under any intellectual property law) be under a kind of good faith obligation that was more extensive than had previously been the case in Europe, and that the EU competition authorities might regard breach of such a good faith requirement as constituting an abuse of a dominant position. That perception is perhaps less prominent now, but at the time such views were widely discussed. A person might therefore have had a concern that a patent proprietor applying for an SPC based on a patent that the proprietor knew or should have known to be invalid, because the reason for invalidity was their own prior art, might be found to be an abuse of a dominant position under competition law.
There is no clear authority that it is an abuse of a dominant position to apply for an SPC based on an invalid patent (even where the patentee should know it is invalid, for example because the prior art is their own related application), but it may well have seemed in 2013 that this was the direction in which competition law was heading. The EU Commission can fine an undertaking up to 10% of worldwide turnover, so the risks involved if competition law comes into play are very high.
By not bringing the SPC into force, it was pretty much guaranteed that there would never be any litigation under the patent, since it would expire before the end of the data exclusivity period. On the other hand, if the SPC were brought into force, there would inevitably be litigation with generic companies, and there would be a risk that the patent might be found invalid for this self-collision reason. Once that reason became public by reason of the litigation, there could then arise a concern that this would lead to EU Commission action under competition law.
I do not know why Pfizer decided not to bring the SPC into force. However, this priority issue leading to a potential self-collision provides a plausible reason (and I have not seen any other possible reason suggested) why someone might have decided that it was too risky to bring the SPC into force. I wonder whether anyone can tell me whether it is anywhere close to the real reason.
Category Chemistry, IP (Intellectual Property), Patents, Science | Tags: patents, pregabalin, supplementary protection certificate
The IP Alchemist
Patent law, chemistry, language and more